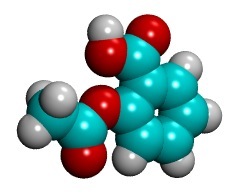
It is made up of 9 carbon atoms (the light blue ones), 4 oxygen atoms (the red ones), and 8 hydrogen atoms (the white ones), put together into this particular shape.
Atoms don't actually look like smooth colorful spheres, more like fuzzy nondescript balls (sort of). But it is easier to see how they fit together this way.
Lots of different molecules exist, from small ones with just a few atoms to large ones with hundreds of atoms. (Can you think of a molecule with lots of atoms? If not, see the bottom of this page for an example of a common one.)
We are interested in a couple of molecules (or molecule fragments) made purely of carbon, that were discovered not that long ago: